P255 Colour in Dentistry: Is Everything We Know Re...


of verifiable CPD
Esthetic restorations represent a significant and increasing portion of dental services and income. Closely related to the...
More Info Purchase CPDAll GDC registrants involved in prescribing, manufacturing and fitting dental appliances have a role to play in protecting patients from harm and in providing a safe and effective standard of care.
This course aims to provide learners with knowledge and understanding of what dental devices are subject to the Medical Devices Directive 93/42/EC (MDD) regulations and your own responsibilities for compliance to the regulations.
This course aims to provide learners with knowledge and understanding of what dental devices are subject to the Medical Devices Directive 93/42/EC (MDD) regulations and your own responsibilities for compliance to the regulations.
On completion of this module you will be aware of what dental devices are subject to the Medical Devices Directive 93/42/EC (MDD) regulations and your own responsibilities for compliance to the regulations.


of verifiable CPD
Esthetic restorations represent a significant and increasing portion of dental services and income. Closely related to the...
More Info Purchase CPD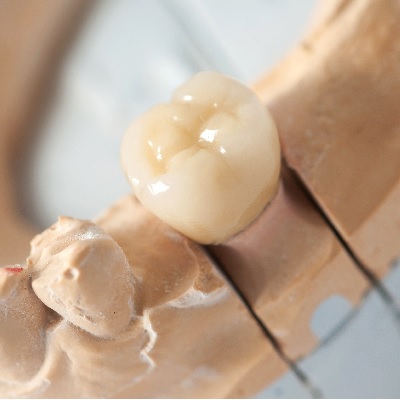

of verifiable CPD
The plethora of ceramic systems available today for all types of indirect restorations can be confusing and overwhelming f...
More Info Purchase CPD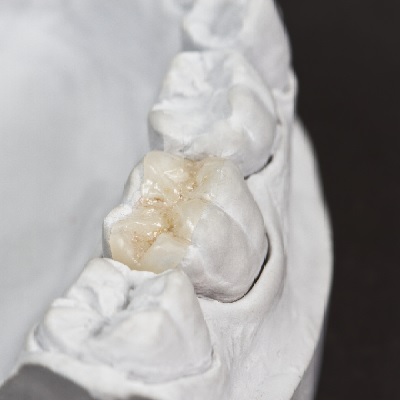

of verifiable CPD
Bonding porcelain restorations can be problematic and time consuming, which has caused many dentists to avoid using bonded...
More Info Purchase CPD

of verifiable CPD
Effective and regular maintenance of equipment in a dental laboratory is essential to the day to day use and efficacy of t...
More Info Purchase CPD